Cluster D. Oncology & Infectious Diseases
Translational Preclinical Pharmacology of Anticancer Drugs
Collaborative Project D1
James M. Gallo, Ph.D. (Project Leader)
Mount Sinai School of Medicine (New York, NY)
David Z. D’Argenio, Ph.D. (Primary BMSR Liaison)
The overall goal of this new Collaborative Project is to apply systems modeling and population analysis approaches to better understand the mechanisms underlying drug action for the treatment of brain tumors. This will be accomplished via the following Specific Aims:
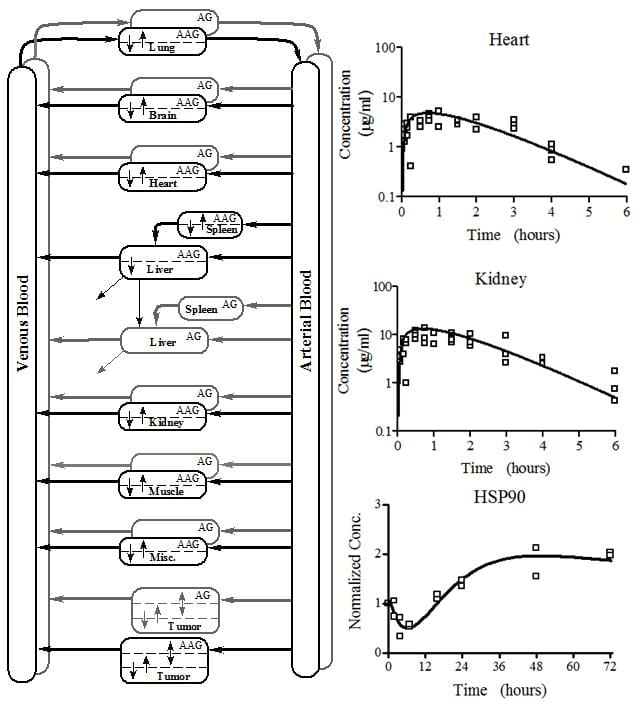
- Develop a systems pharmacology model linking the in vivo PK of a series of epidermal growth factor receptor inhibitors, to cellular tumor pharmacodyanamics, and to tumor burden in a murine model.
- Develop a physiologically-based pharmacokinetic model for combination therapy involving both angiogenesis inhibitors and cytotoxic drugs in a rat glioma xenograft.
- Using population modeling methods develop of a composite model of methotrexate kinetics focusing the mechanisms of hepatic metabolism, biliary excretion and renal elimination and the role of transporters.
Investigational Anticancer Agents
Collaborative Project D2
Jan H. Beumer, Pharm.D., Ph.D. & Julie Eiseman, Ph.D. (Project Leaders)
University of Pittsburgh, Cancer Center (Pittsburgh, PA)
David Z. D’Argenio, Ph.D. (Primary BMSR Liaison)
The overall goal of this project is to provide a comprehensive evaluation ((molecular, biochemical, cellular, animal, and clinical) of the pharmacokinetics and pharmacodynamics of investigational antineoplastic agents in preclinical, clinical Phase I and multi-center Phase II/III trials. This project also aims to develop approaches that improve the overall efficiency of the drug development process, including translation of preclinical and Phase I results to clinical trials. Currently the following aims are addressed:
- Study the pharmacokinetics and pharmacodynamics of the novel indenoisoquinoline topoisomerase 1 inhibitor, LMP400, administered on a daily x 5 schedule.
- Veliparib is a PARP inhibitor undergoing extensive clinical evaluation in glioblastoma, because it may synergize with the standard-of-care temozolomide. We will examine the important factors controlling veliparib efficacy in glioblastoma.
- 25-hydroxyvitamin D3 (25(OH)D3) levels are associated with increased overall survival in early stage non-small cell lung cancer (NSCLC), and it has been found to be converted locally in tissues by CYP27B1 into its active metabolite. This aim will investigate the role of CYP27B1 in mediating the anti-tumor effects of 25(OH)D3 in NSCLC.
Treatment of Hepatitis-C and HIV Infections
Collaborative Project D3
Jennifer J. Kiser, Pharm.D. (Project Leader)
University of Colorado, Boulder (Boulder, CO)
David Z. D’Argenio, Ph.D. (Primary BMSR Liaison)
The overall goal of this new Collaborative Project is to apply systems modeling and population analysis approaches developed in Core Project #1, to better understand the mechanisms underlying existing approaches for treating chronic Hepatitis C virus (HCV). This will be accomplished via the following Specific Aims:
- Develop a model for cellular metabolism of ribavirin that links the plasma concentration of the drug to the intracellular pharmacokinetics of the phosphorylated anabolites of ribavirin that mediate its antiviral and toxic effects.
- Using the covariate imbedding approach to population modeling, develop a complete population model of plasma and intracellular ribavirin in an effort to identify informative covariates
- From the population model, identify response and toxicity relationships relative to plasma and intracellular ribavirin exposures.
Antiviral and Antiretroviral Drug Therapy
Collaborative Project D4
Edward P. Acosta, Pharm.D. (Project leader)
Univ. of Alabama, Birmingham, School of Medicine, (Birmingham, AL)
David Z. D’Argenio, Ph.D.(Primary BMSR Liaison)
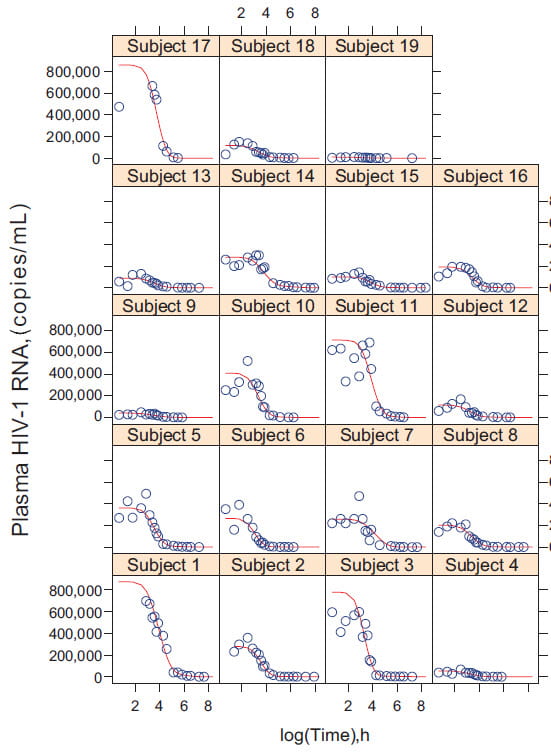
The antiviral pharmacology laboratory focuses on antiviral assay development for Phase I-II trials with the aim of determining optimal doses, dosing schedules, and linking activity to drug exposure using complex PK/PD modeling approaches. Current specific aims include:
- A Phase II study of oral valganciclovir versus placebo in infants will be conducted to evaluate antiviral treatment of infants with hearing loss related to congenital cytomegalovirus infection.
- A pharmacokinetic/pharmacodynamic and resistance evaluation of intravenous ganciclovir in premature infants will be used to evaluate the antiviral drug dosing in extremely premature infants with congenital or postnatal cytomegalovirus disease.
- A multiple ascending dose-finding pharmacokinetic and pharmacodynamic study of CMX-001 in infants with neonatal herpes simplex virus will be conducted to evaluate this novel antiviral drug for the treatment of neonatal herpes simplex virus disease involving the central nervous system.